Geninus has developed NGS-based liquid biopsy analysis technology on the basis of clinical trials. A global level of accuracy has been secured by securing the noise removal effect through use of technology capable of identifying circulating tumor DNA (ctDNA) with tumor-specific somatic cell mutation with high sensitivity and specificity. This was achieved by executing analysis of cell-free DNA (cfDNA) in blood with targeted in-depth sequencing capable of removing sequencing errors, as well as through use of an algorithm to detect genome mutations with high sensitivity and specificity in small quantities of DNA.
Liquid biopsy-based cancer genome analysis technology can be performed through simple blood collection, which enables early-stage diagnosis and treatment of cancer and recurrence monitoring. Moreover, it can be used across every stage of clinical trial and for selection of customized treatment drugs.
-
01In-House Molecule Barcoding Technologies
Since the quantity of cfDNA in a blood sample is very small in the case of a liquid biopsy compared to a tissue biopsy, very high levels of sensitivity and specificity are required in order to detect genome mutation. As such, it is particularly important to reduce PCR errors that occur in the process of library production. Our company has suppressed PCR error occurrence through application of our own molecule barcoding technology and applied molecule barcoding structure innovation technology to reduce the adaptor switching phenomenon that manifests in the process of molecule barcoding.

-
02Machine Learning-Based Noise Filtering Technologies
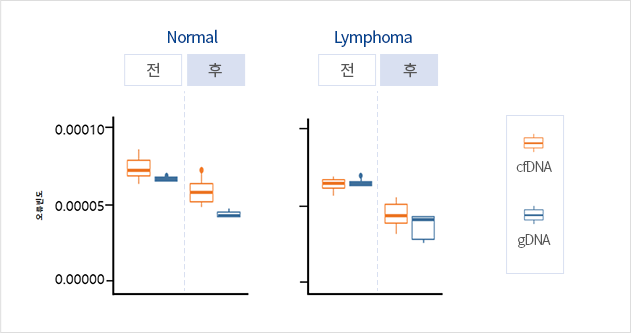
We have improved sensitivity and specificity by distinguishing the noise of cfDNA sequencing data from mutation signals through machine learning with approximately 1,000 internal data cases accumulated in our in-house database. Results of clinical verification of normal specimens and lymphoma specimens confirmed a maximum of 42.7% error suppression when comparing prior to and following algorithm application.